Strategic Planning
Following an analysis of the internal and external scenario, in 2022 Aché strategic plan was updated to capture the main trends and opportunities and identify the potential disruptions in the health ecosystem that may impact the business. We established our strategic drivers to reinforce the Company’s performance in a management model that is structured in line with our transversal approach to ESG. We maintained our objective of ensuring the Company’s competitive position and the continuity of our business model, and we strengthened and expanded our operations in the health segment.
Strategic Drivers

A leader in prescription drugs
A leader in
innovation

Relevance to customers
Operational efficiency
Asset maximization

Digital transformation and cultural evolution
Sustainability Strategy
GRI 3-1 | 3-2 | 3-3
Materiality
GRI 3-1 | 3-2 | 3-3
We are convinced that Aché’s continuity is closely linked to our sustainability strategy, which guides the business decisions we make. We have, therefore, reviewed our materiality matrix to ensure that the relevant environmental, social and governance issues are properly mapped out and reflect our stakeholdersexpectations, trends, risks, and opportunities.
To identify these issues, we have gathered aspects of the organizational culture, strategy and business vision through interviews with shareholders and the Board of Directors, as well as surveys with the executive board and internal and external stakeholders. Impressions were collected from more than two thousand stakeholders, including employees, physicians, points of sale, suppliers, consumers, third parties, clinics and hospitals, distributors, journalists, public sector organizations, trade associations, and other audiences.
Consolidation of the material themes was carried out by crossreferencing the information, considering the vision of the stakeholders and the Company’s internal vision, covering the priority focuses of different stakeholders.
Having identified and consolidated these views, we arrived at Aché’s 14 material themes::
1. Product Safety and Quality
2. Data Privacy
3. Occupational Health and Safety
4. Talent Attraction and Retention
5. Diversity, Equity, and Inclusion
6. Human Rights
7. Waste Management
8. Ethical Behavior
9. Biodiversity
10. Access to Health Care
11. Climate Change
12. Supply Chain
13. Eco-Efficiency
14. Risk Management
Material Themes and Priority SDGs
Aché's business model and purpose have a connection intrinsic to sustainability, as the products that we develop help promote the health and well-being of millions of people. We understand and assume our responsibility.
That is why, over the years, we have consolidated a corporate culture based on the development of competitive businesses, with solid economic results, and that, at the same time, identify, neutralize or mitigate their socio-environmental impacts.
In 2021, we progressed on our sustainability journey. Based on recognized criteria, we undertook a broad diagnosis of the whole organization, our objective being to map our maturity and identify opportunities for improvements in environmental, social and governance issues. Following the results of the evaluation, we modified Aché’s sustainability management structure, which now has an ESG Executive Committee, which is linked to the Company’s Administrative Council, and a Sustainability Board that reports directly to the CEO. We also set up a Sustainability Department
Based on the above diagnosis, our new materiality matrix and our organizational purpose, we developed our sustainability strategy in 2022, material themes and drivers, including the Sustainable Development Goals (SGG).
The following were defined as priority SDGs for Aché:
ACHÉ’S ESG ACTION FRONTS
Commitments
GRI 3-3
We devised and set out our 2030 agenda, which establishes our short, medium, and long- term commitments. Our goal is to continuously contribute to solving global challenges while acting locally using Aché’s business model. The criteria, indicators and the baseline will be established in 2023.
The short, medium, and long-term commitment agenda includes the following objectives:
• Zero the sending of industrial waste to landfills;
• 100% of electricity comes from a renewable base;
• Implement and certify environmental, energy, and occupational health and safety management systems in all industrial units;
• To achieve climate neutrality;
• To protect Brazilian biodiversity;
• To improve our operational eco-efficiency: water resources and packaging:
• Having an environment with diversity, equality, and inclusion: commitments to PWD, 50+, Ethnicity, Gender, Pay Equity, and LGBTQIA+;
• Promover a transparência junto a stakeholders: the structuring and dissemination of KPIs of material themes
• To influence and monitor our supply chain: ESG criteria as part of the approval, contracting, monitoring and consequence management processes.
In 2022 we issued a second tranche of simple debentures linked to our sustainability goals, and which are called Sustainability-Linked Bond Principles (SLBP), which were created by the International Capital Market Association (ICMA) in June 2020. The debenture reinforces the Company’s transparency and its accountability to investors and other stakeholders.
Expansion of presence
Opportunities for the Aché Generation
With the creation of the Biosynthetic Prescription Unit, the Company generated 279 vacancies. Almost 50% of these vacancies were filled by internally promoted and relocated employees.

Innovation and Biodiversity
GRI 2-6 | 3-3
Innovation is one of Aché’s strategic drivers. Based on culture and demonstrated in the products, services and processes, that contribute to the Company’s continuity, this is one of the main drivers of our business.
Among the main investments are:
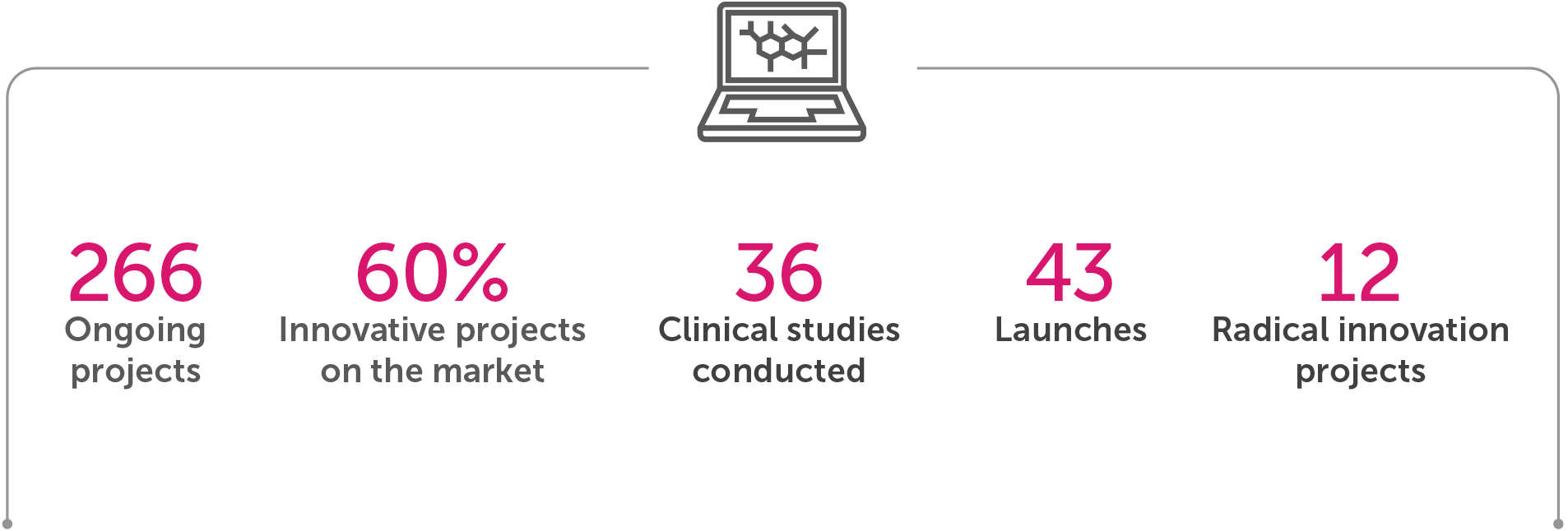
• Pipeline with 266 projects under development;
• Radical innovation with 12 projects eight of them coming from Brazilian biodiversity; and
• Important advances in clinical studies: with the completion of 36 studies.
QUALITY AND SAFETY IN CLINICAL TRIALS
Aché conducted various clinical studies in Brazil in 2022 and maintains its responsible and ethical posture in full compliance with good clinical practices (GCP), thus ensuring strict compliance with the study protocols that are duly approved by the Research Ethics Committees accredited by National Research Ethics Commission (Comissão Nacional de Ética em Pesquisa - CONEP). The Company has designated research monitors who supervise the progress of each clinical study and ensure that they are conducted in accordance with the study protocol, with the standard operating procedures of Aché and the research center, and with GCP and other applicable regulatory requirements. During monitoring visits, the monitors aim to ensure the integrity of clinical data and the protection of the rights and well-being of research participants.
Aché’s pharmacovigilance and medical teams and the research centers evaluate any adverse event (any unfavorable medical occurrence that may happen during the study, but that does not necessarily have a causal relationship with the treatment) that is observed by health professionals or spontaneously reported by research participants. Aché’s pharmacovigilance team monitors any sign that may lead to an alert or risk involving the safety of participants in order to ensure their well-being. The Company provides immediate and comprehensive assistance, if or when necessary.
Incremental Innovation
The incremental innovation front uses pharmaceutical technology applied to existing molecules in order to create new products aimed at the unmet needs of prescribers and patients. In 2022, we inaugurated our InnovatechLab, a laboratory dedicated to the research and development of new technological platforms that will allow Aché to create innovative products in Brazil and worldwide, in partnership with research centers and universities.
The InSPIre Lab (In Silico Prediction, Information & Research Lab), which was inaugurated in 2020, analyzes large-scale data (Big data) and uses artificial intelligence (AI) to enrich the ideation of new products to be developed in-house and to seek innovative products around the world, which will be brought to Aché through strategic partnerships. It also builds the tools that make it possible to streamline the innovation process.
To ensure agility and accessibility in innovation and the renewal of its portfolio, Aché has a robust infrastructure of processes and technological platforms, and specialized teams that work with incremental and radical innovation in the Company
Radical Innovation
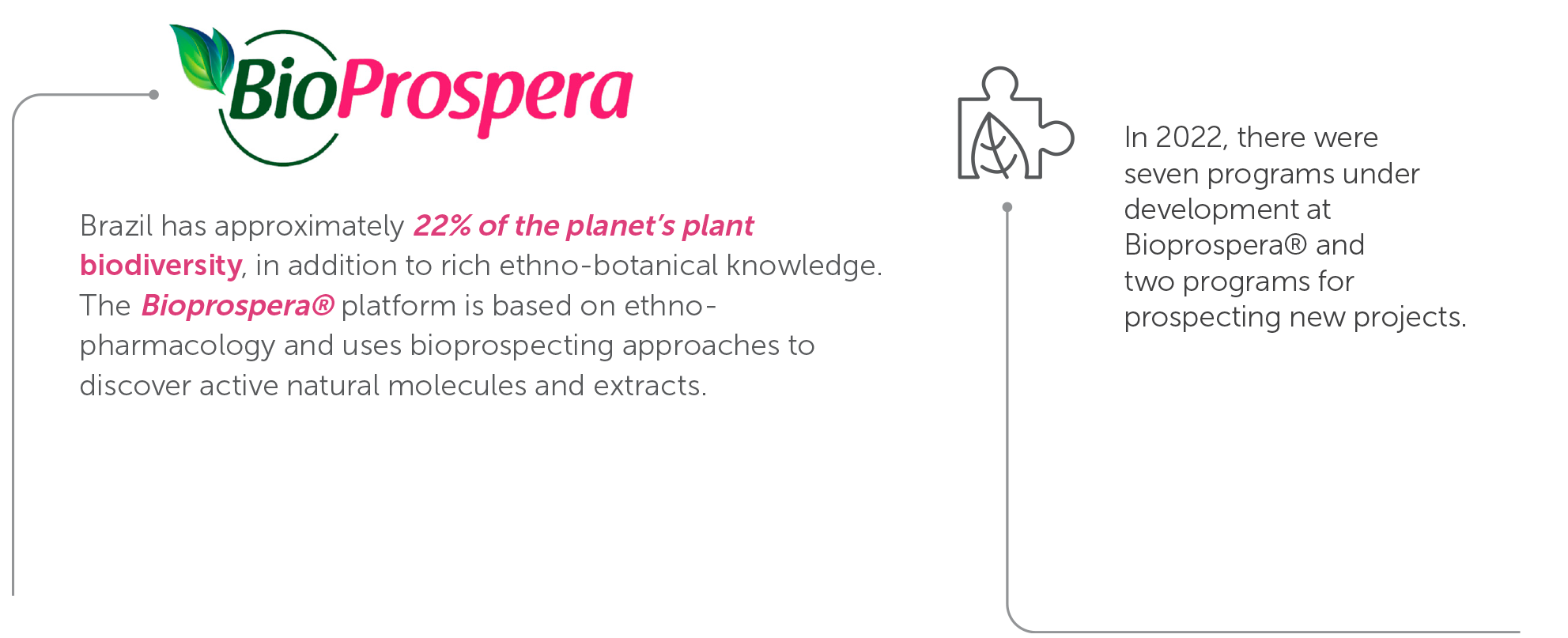
Currently, the company is developing radical innovation projects, also related to Brazilian biodiversity. To discover and develop innovative medicines from natural sources, especially from Brazil’s biomes, the Bioprospera ® platform projects are highlights in innovation at Aché. The platform was structured to be sustainable and follows ESG principles when delivering therapeutic solutions to bring a better life to people and improved social development, thus creating value from biodiversity.
Since 2021 Aché has been part of a prospecting program in the Amazon region. The program was expanded in 2022 to identify potential projects in all Brazilian biomes. The results of this prospecting in 2023 will enable advances by Aché and startupsA development from this initiative was the selection of nine technologies with the potential for use in future partnerships.
The Molecular Design and Synthesis Laboratory develops therapeutic solutions through research into new molecules that can revolutionize treatments Thousands of molecules have been synthesized for the Company’s different projects since 2015.
Aché has established important partnerships, such as the project with Phytobios and the National Center for Research in Energy and Materials (CNPEM), where the 4th generation particle accelerator (Sirius), one of the most advanced in the world and extremely important for the characterization of natural bioactive molecules, is located.
A reference in pharmaceutical innovation, for the 8th consecutive year the Company has won 1st place in the Pharmaceuticals and Life Sciences category in the Brazil Innovation Value (Valor Inovação Brasil) 2022 Awards, which evaluates the innovation practices of companies operating in Brazil in different sectors.
Para estimular a prospecção de parcerias com empresas inovadoras, o Aché possui um hub of startups que desenvolvem projetos focados em solucionar desafios do segmento farmacêutico.
We have partnerships with both public and private universities for developing innovation projects.
Public Universities:
• FMABC – ABC School of Medicine
• Unicamp – State University of Campinas
• USP - University of São Paulo
• Santa Casa de Misericórdia de São Paulo
• LNBio – National Laboratory of Biosciences
• Unifesp – Escola Paulista de Medicina
• Unesp Botucatu – Paulista State University
• Ribeirão Preto Medical School of USP
Institutions linked to private universities:
• São Francisco University in Bragança Paulista
• Sociedade Beneficente Israelita Brasileira Hospital Albert Einstein
Digital Transformation
GRI 2-6 | 3-3
Through digital transformation, Aché has consistently advanced in its efforts to become increasingly relevant to customers and other stakeholders, and has succeeded in executing its strategy. Last year we invested BRL 157.5 million in strategic projects in digital transformation and information technology. In addition to investments that focus on the digital front and the modernization of our technology park, the company has evolved in its journey in cloud computing, cybersecurity, and its data structure, making way for the consolidation of an analytics structure.
Together with these initiatives and in line with the Leadership in Innovation driver, we have consolidated our open innovation program, which seeks to establish an approach to entrepreneurs with the objective of evaluating and internalizing market solutions that can solve operational challenges and leverage new business for the Company.
Another important milestone was the creation of the Digital Products team that focuses on developing solutions for our employees, patients, doctors and points of sale. An example of the use of these products is in accelerating the recruitment of participants and conducting clinical studies better. Another example is the adoption of the e-TMF system in the clinical development area, which ensures the security of the documentation generated in the studies and enables information to be tracked.
The use of digital platforms for the relationship between health professionals and patients has expanded and has exerted a direct influence on our scientific communication with these professionals. Aché has diversified this form of communication by using podcasts, video classes, pocket videos, hybrid materials (written/video) and, whenever possible, social networks for our publicizing product and communication activities.
Internally, the Company has digital communication channels to stay close to employees, disseminate the Aché culture, publish relevant information about the Company’s strategy, train topics that are regulated by corporate policies and foster the engagement of everyone to fulfill our purpose of bringing a better life to people.
Excellent Performance
GRI 2-6
The year 2022 was marked by major challenges, the result of a macroeconomic context of high inflation, high interest rates, the typical uncertainties of an election year and instability in our production costs. In addition to these factors, continuing outbreaks of Covid, influenza and other respiratory diseases generated high demand and challenged service provision due to bottlenecks in the supply of pharmaceutical inputs and excipients. The resilience of the pharmaceutical market, however, resulted in a 17.1% growth in PPP demand in the Retail Channel compared to 2021, which was slightly higher than the market according to data released by IQVIA*.
In line with market trends and to promote access to health, Aché explored the positive factors that had a strong influence last year, such as increased demand for respiratory products, adherence to telemedicine and the of medicines via e-commerce .
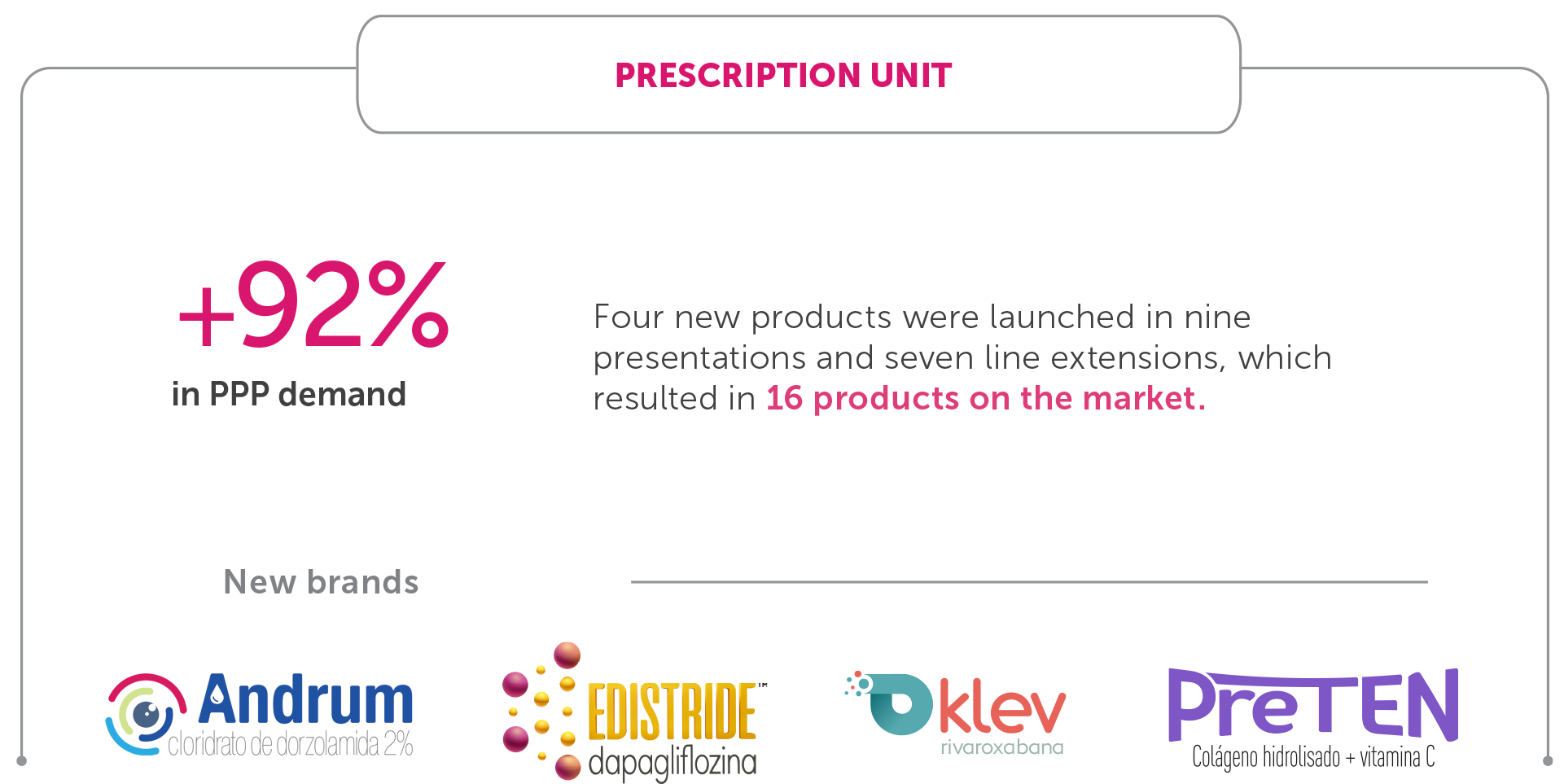
Prescription Division – Prescription Business Unit
The Prescription Unit has a significant participation in the Company’s activities and accounts for more than 70% of our total revenue. In 2022, the respiratory diseases segment grew by 21.9% and contributed to the 21.7% increase in the unit’s net revenue compared to 2021. The Edistride and Klev launches, in the Cardiometabolism Franchise, Andrum in Ophthalmology, and Preten in Osteomuscular made a relevant contribution to new product revenue.
The work of the teams that participate in the sales force, productivity, marketing, and other areas that are part of the cycle that places a product on the market contributed to the success we achieved. They did this by way of the strategies we implemented in services, solutions, training and virtual medical education and the online promotion tools we used, all of which leveraged our performance last year.
In 2022, Aché received 14 magnifier, which recognize the best campaigns and marketing professionals in the pharmaceutical industry.
We expanded the content available to physicians on the Aché.doc portal through classes and scientific information. We held more than 250 webinars and arranged WhatsApp visits and the sample showcase. These innovations strengthen the relationship between our sales reps and physicians.
Prescription Division – Institutional Business Unit and Special Care
Institutional and Special Care Unit works with highly complex therapies in the areas of Oncology, Hematology and Critical Care and markets the entire Aché portfolio to public and private institutional clients, such as hospitals, clinics and public agencies (Ministry of Health, state secretariats and city halls). This demand has increased consistently over the past two years due to product launches and Aché’s efforts to promptly meet the new demands of hospitals. In 2022, the unit’s net revenue grew by 28.6%, driven by the launch of our Volare, Lessav and Capzat products.
Expansion of the Demand, Access and Institutional Sales teams was fundamental for increasing the commercialization of our products and contributing to public health and the well-being of society.
Aché obtained approval to indicate the drug Decadron for the treatment of Covid-19 on the package insert.
The treatment is indicated for patients with severe or critical conditions of the disease. Anvisa’s approval was based on the recommendations of the World Health Organization (WHO) published in the document, “Therapeutics and COVID-19: a living guideline”, and on the manifestations of equivalent foreign regulatory authorities, in particular the European Medicines Agency (EMA).
Consumer Health Division
The Consumer Health Division consists of the Trade Marketing area and three business units: Prescriptionfree Drugs (MIP), Generics, and Dermatology. Together, these units represent 22% of Aché’s total net revenue. In 2022, the Consumer Health Division grew 23.5% in PPP demand.
This year, in which respiratory diseases and cold and flu outbreaks prevailed, Aché fulfilled its role of bringing more life to people, with solutions such as Decongex, which almost doubled in size, reaching BRL 337M in PPP demand, consolidating itself as the 7th largest brand in the Brazilian MIP market.
Driven by the driver of our strategy, Customer Relevance, we launched the PEAS, which is the Prescription Drug Shopper Service Excellence Program. By means of data from several surveys and performance indicators, we support retailers to properly position their over the counter service and thus gain the loyalty of their customers.
Consumer Health Division – MIP Business Unit
MIP gained market share this year due to demand from the flu and cold categories and grew by 61% compared to 2021.
We optimized the promotional mix between media, medical and trade marketing with a 360-degree operation in the three channels, and executed the strategy of accelerating the major brands. We drive brand awareness through the mass media and impact millions of people each year.
Generics unit
By maintaining consistency in strategy, we achieved a 3.4% market share, ranking 9th in the market.
The year saw volatility in demand, due to insecurity in the supply chain. Thus, the strategy of increasing the focus on the sales and demand areas brought a relevant contribution to the unit’s result, which even though it suffered from challenges in forecasting and meeting volume, delivered more than double the contribution margin. The result was also supported by the product mix and cost management.
Consumer Health Division – Dermatology Unit
Aiming to maximize each product’s potential, the Dermatology Unit underwent structural changes in 2022. Dermomedicines became part of the Prescription Division - Prescription Unit, with expanded operations for other specialties besides Dermatology. The restructuring of the Dermatology Unit made it possible to give full focus to Dermocosmetics, which remained in the Consumer Health Division. With a 12-year history, the Profuse brand has gained prominence among dermatologists, ranking as the 5th most prescribed brand. Following the restructuring, Profuse now has a dedicated medical advertising team, which visits dermatologists specializing in beauty and well-being.
Operational Efficiency
GRI 2-6 | 3-3
Continuing the process of excellence already established in the Company, we developed new initiatives to optimize costs and production capacity in the operations areas. Investments in manufacturing facilities, including the Pernambuco plant, the pilot plant, the modernization of our manufacturing parks, the acquisition of new equipment and improvements in the layout of our operations areas, amounted to BRL 255.5 million.
The maintenance plans of the plants involved routine inspections and preventive maintenance, for which we formed improvement groups and trained more than 300 employees in the concepts of Yellow Belt Lean Six Sigma. Digital platforms helped improve the maintenance area. Data were obtained via the Centerline platform for predictive maintenance and the efficiency of our equipment and machinery in producing medicines.
Our plants are structured to reach excellence in execution and to be quick in product availability and delivery, eliminating losses and increasing performance and production. To this end, we have produced 266 million units last year.
At the Pernambuco plant, there was a monthly record of 12.5 million units produced, while on the production line of medicines related to colds and flu, the medication Decongex, reached the milestone of over 1 million units produced in a single month. Aché’s total installed capacity, considering its five plants, is 626 million units.
We completed Phase 2 of the Pernambuco plant with the construction of the Solid Manufacturing building and the Quality Control Laboratory, and we started producing medicines.
During the year we optimized the process by which the production stages of our Pernambuco plant are supplied with inputs. To do so we used automated guided vehicles (AGVs), which are smart vehicles that move cargo within the plant without the need to be controlled by humans, and “stacker cranes”, which are used for all movements within the vertical warehouse and for storing the products according to systemic parameters and in line with good manufacturing practices.
One of the drivers of our operational progress is the Operational Excellence Program (PEO, Programa de Excelência Operacional), which was implemented in the Company in 2015 and that uses the TPM (Total Performance Management) methodology to provide the necessary support for the operations areas. In 2022, the PEO resulted in a reduction of BRL 9.2 million in costsThe program establishes management routines, and improvement and agility projects in the quality area. It is also responsible for the Education & Training pillar, which supports employee training in quality and operational efficiency. PEO has been evolving more and more in the Company, and reinforces our culture of continuous improvement, discipline and agility.
The teams in the five industrial plants sought to develop and train employees to increase their efficiency and reduce waste. The result was increased operational efficiency, in addition to reducing the amount of organic waste sent to landfill sites, reducing the waste of plant pallets and improving our impact on energy consumption. The Guarulhos (SP) industrial plant, which is already ISO 14001 (Environmental Management System) and ISO 45001 (Health and Safety Management System) certified, received ISO 50001 certification in 2022, a result of our investments in efficient energy management.
The certification encouraged the standardization of our energyrelated metrics and objectives and enabled us to have a conscientious view of the use of this resource.
Product Quality and Safety
GRI 2-6 | 3-3 | 416-1
Fulfilling our purpose of bringing a better life to people by way of quality, efficacious and safe products, Aché performs quality tests on the whole of its portfolio. Evaluations are carried out in accordance with national and international standards
The Aché Quality System follows strict international criteria and standards. In recent years, a reformulation of the Company’s quality structure has been carried out in order to increase productivity, which has resulted in progress being made in terms of our operational excellence and a greater technical focus for dealing with demands. The five plants were cohesive in their management and processes in line with good manufacturing and laboratory practices
Our Customer Service Center – CAC (Central de Atendimento a Clientes), which handles all in-coming calls related to Aché’s products and distributes them to the areas responsible for dealing with them, also works with the Pharmacovigilance area, which evaluates and monitors the safety data informed by health professionals and consumers. The feedback we receive is periodically reported to the National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária - Anvisa), in line with current legislation. The monitoring of possible adverse events after our products are sold is carried out continuously, ensuring that any possible risks arising from their use are mitigated.
We have an exclusive medical information channel for health professionals via WhatsApp, which is part of our Sales Force system, which disseminates quality information to this public.
Regulatory Environment
GRI 2-6
We operate with respect for and compliance with the sector’s legislation and regulatory bodies, with which we closely work to achieve a high level of quality and safety. Through dialogue and partnerships we strive to strengthen an innovative national industry
In 2022, Anvisa approved a new milestone in the process for registering new and innovative drugs through RDC 753/2022 and Normative Instruction 184/2022, which will allow the population to have greater access to new drugs more quickly.
Financial Performance
GRI 2-6 | 201-1
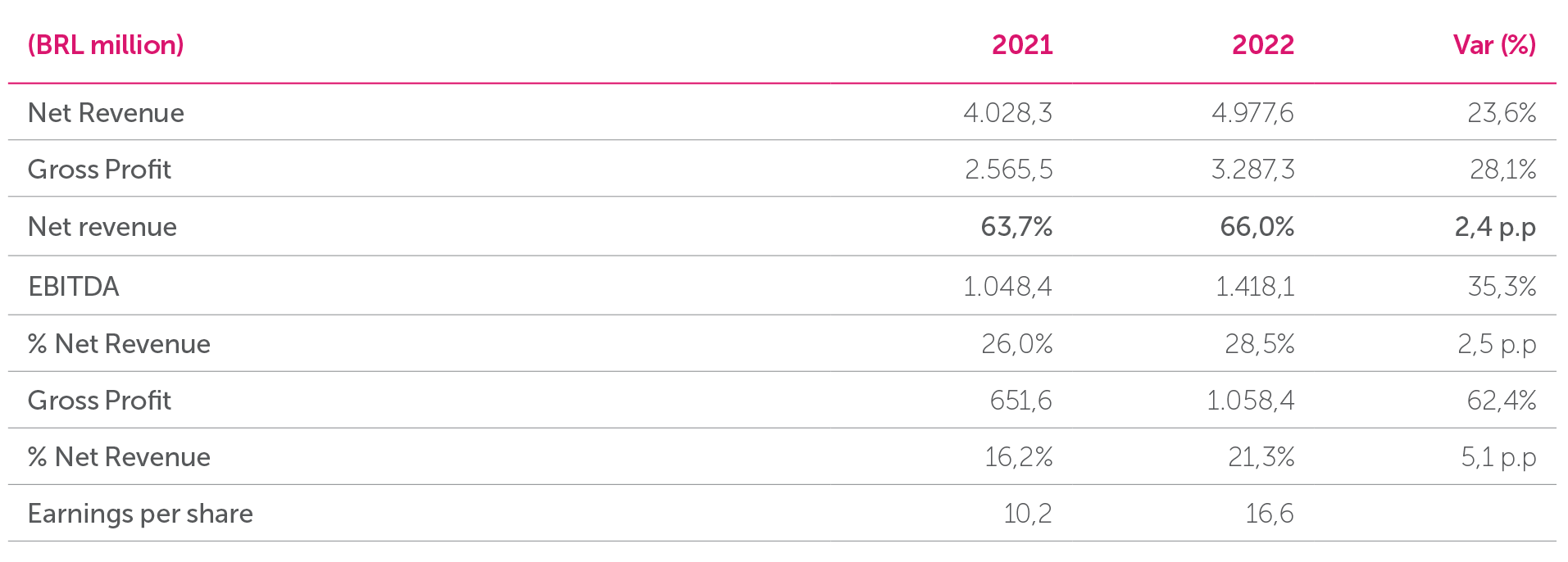
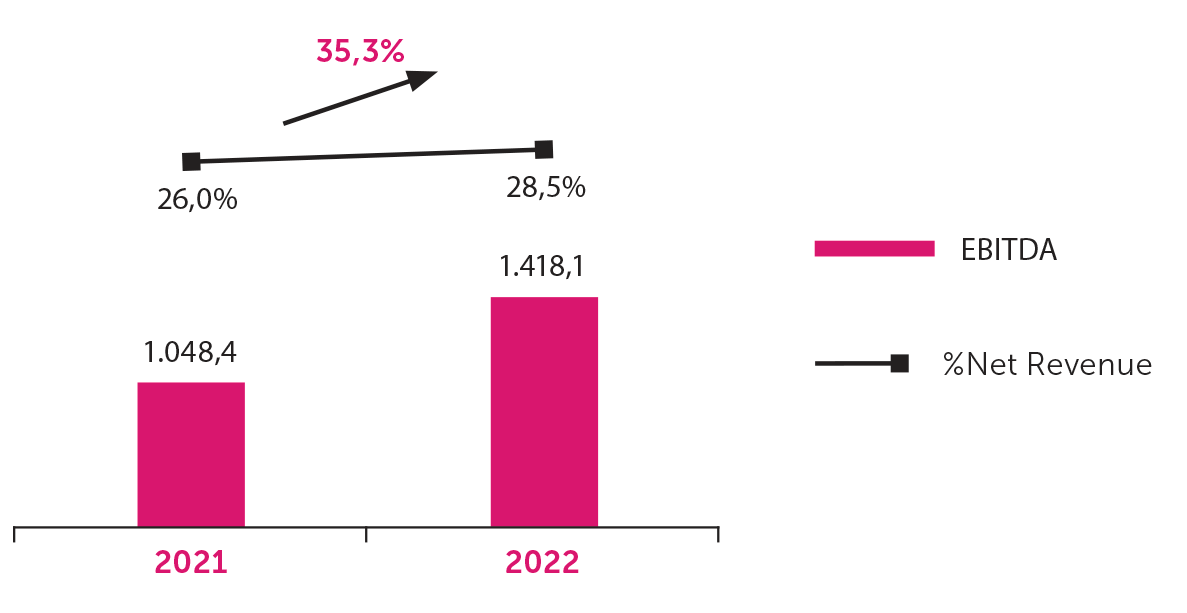
Aché grew more than 23% in 2022, with net revenue of BRL 4.97 billion, thereby highlighting the correctness of our Strategic Plan and the continuity of the business. EBITDA (earnings before interest, taxes, depreciation, and amortization) reached BRL 1.41 billion, 35.3% higher than in 2021. The EBITDA margin was 28.5% in 2022 compared to 26% in 2021.
Net income for 2022 was BRL 1.05 billion, an increase of 62.4% compared to the same period in the previous year, which was mainly a reflection of the increase in net revenue of 23.6%, the increase in the gross margin, and a reduction in the tax burden.
The economic value added (indicator of the wealth added to society by the Company in its economic activity) totaled BRL 2,915.4 million.
KEY FINANCIAL INDICATORS - CONSOLIDATED
Note: Numerical data are in portuguese.
CONSOLIDATED RESULT
Note: Numerical data are in portuguese.
Net Revenue
In 2022 the Company’s net revenue increased by 23.6% in relation to the previous year, which is a reflection of the positive performance in all our business units, particularly the Respiratory and Central Nervous System items of the Prescription Unit, launches of the Special Care Unit, and the evolution of our Generics.
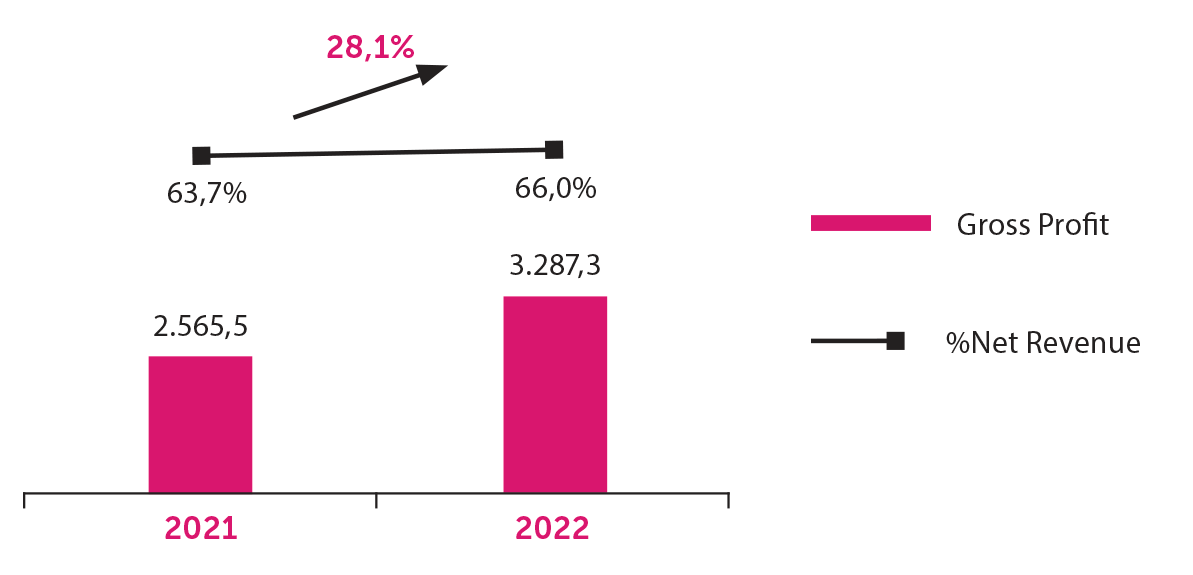
Gross Profit
Gross Profit in 2022 was BRL 3,287.3 billion, 28.1% higher than the previous year, reaching a 66.0% margin on net revenue.
Note: Numerical data are in portuguese.
Sales, General and Administrative Expenses
Sales, general and administrative expenses increased by BRL 306.2 million, compared to the same period in the previous year. This was mainly due to an increase in expenses incurred as a result of new business development, and to promotional and sales expenses that are an inherent factor of the Company’s revenue growth.
Note: Numerical data are in portuguese.
EBITDA
EBITDA (earnings before interest, taxes, depreciation, and amortization) reached BRL 1.418,1 million, an increase of 35.3% compared to the same period in the previous year. This was mainly impacted by the increase in net revenue of 23.6% and the tax benefit from the government subsidy.
Note: Numerical data are in portuguese.
Financial Result
The year-to-date net financial result was a loss of BRL 135.2 million, BRL 64.9 million more than the previous year, mainly due to the provision for interest on the debentures that were issued in August 2022, and an exchange variation, which was mitigated by higher financial income resulting from the interest rate hike and greater availabilities.
Note: Numerical data are in portuguese.
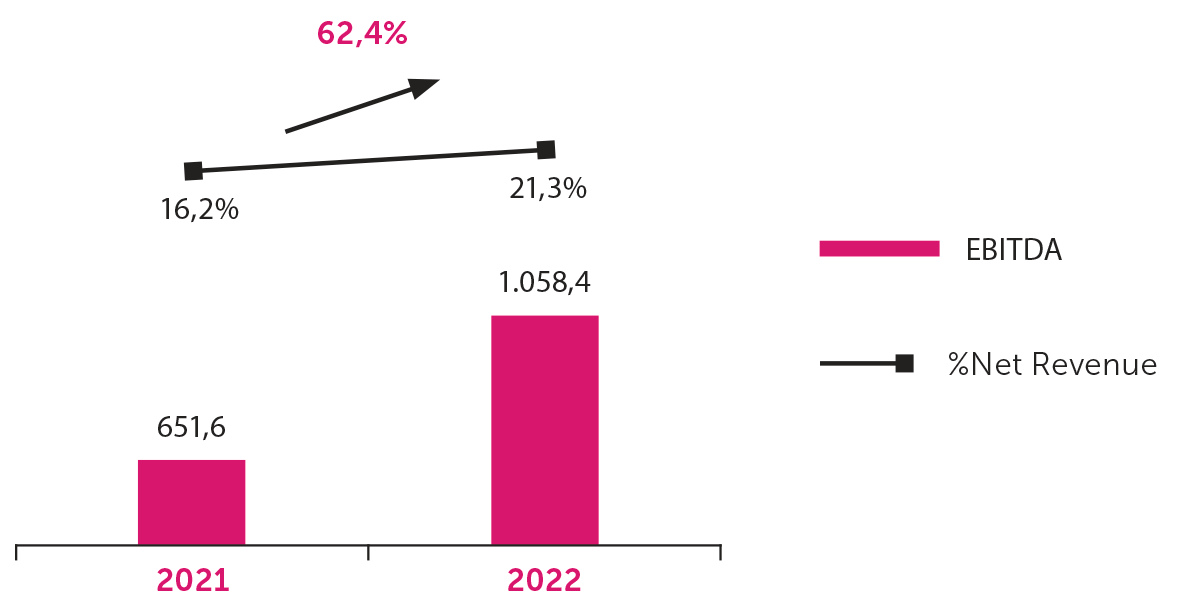
Net Profit
Net profit for 2022 was BRL 1,058.4 billion, an increase of 62.4% compared to the same period in the previous year, reflected mainly by the increase in net revenue of 23.6%, the increase in gross margin and a reduction in the tax burden.
Note: Numerical data are in portuguese.
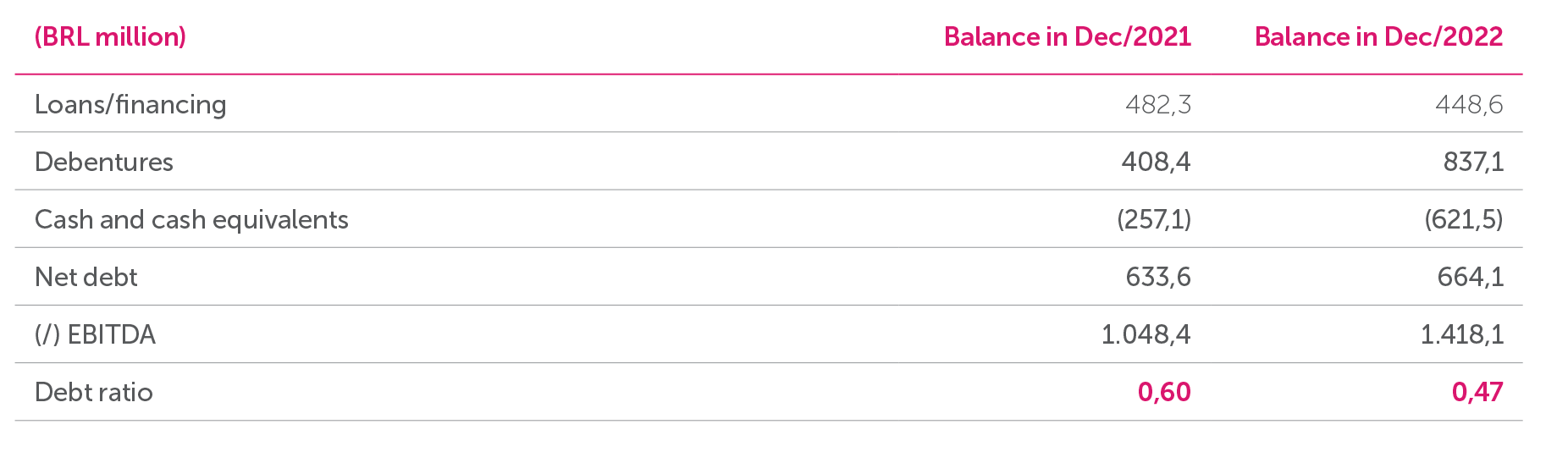
Net Debt
The Company ended the year 2022 with a net debt of BRL 664.1 million. Gross debt totals BRL 1,285.6 million and is mainly long term.
Note: Numerical data are in portuguese.
The variation in the gross debt is substantially related to the debenture issue in August 2022 in an amount of BRL 400 million, he funds from which resulted in a higher level of cash and cash equivalents that will be allocated to the regular management of the Company’s operations.
Debt Ratio
The Company has financial instruments that must comply with indebtedness limits (Covenants). In 2022 the Company is compliant with these contractual undertakings.
Note: Numerical data are in portuguese.
Value-added statement
The value added, which is an indicator of the wealth added to society by the Company from its economic activity, totaled BRL 2,915.4 billion. The full statement can be found in the financial statements.
Note: Numerical data are in portuguese.
Investments in Fixed Assets
Investments in 2022 in fixed assets totaled BRL 257.0 million, of which BRL 119.6 million were used in implementing the new plant and modern vertical warehouse in Cabo de Santo Agostinho - Pernambuco (BRL 146.6 million in 2021).
Dividends and interest on own capital
The Company’s Bylaws ensure a minimum mandatory dividend corresponding to 25% of each year’s net profit, and allow the distribution of dividends based on half-yearly or interim balance sheets.
Note: Numerical data are in portuguese.
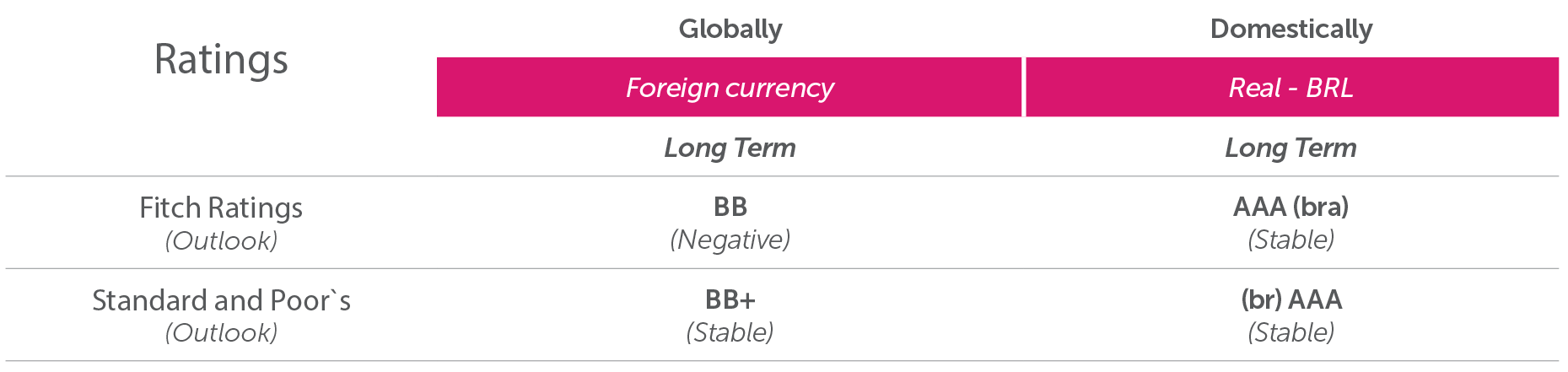
Rating
Maintenance of the ratings by Fitch and Standard & Poor’s reflects Aché’s strong business position with its outstanding participation in the prescription drug segment and well-established brands, in addition to the reach of its extensive sales force, which provides it with a competitive advantage. The ratings also incorporate the low risk of the Company’s product portfolio, which has no material exposure to patents and licenses, and its commitment to an unfavorable financial leverage capital structure while managing its growth plans with resilient and free cash flow generation.